At CLINWA, our innovative approach includes adaptive trial methodologies.
Our project managers, clinical research associates, and other members of the team know that these protocols require more than simple analyzing lab draws before and after drug doses; they also involve a human review process.
Our expertise in clinical trial management will ensure the success of your trial – helping you bring new products to market safely and quickly and prevent hurdles, such as inexperienced resources, inefficient feasibility and under-estimated start up.
We offer you:
Protocol Development
Feasibility
Start-Up
Contracts and Budgets
Vendor management setup
Risk Management
Data Management
Regulatory Affairs
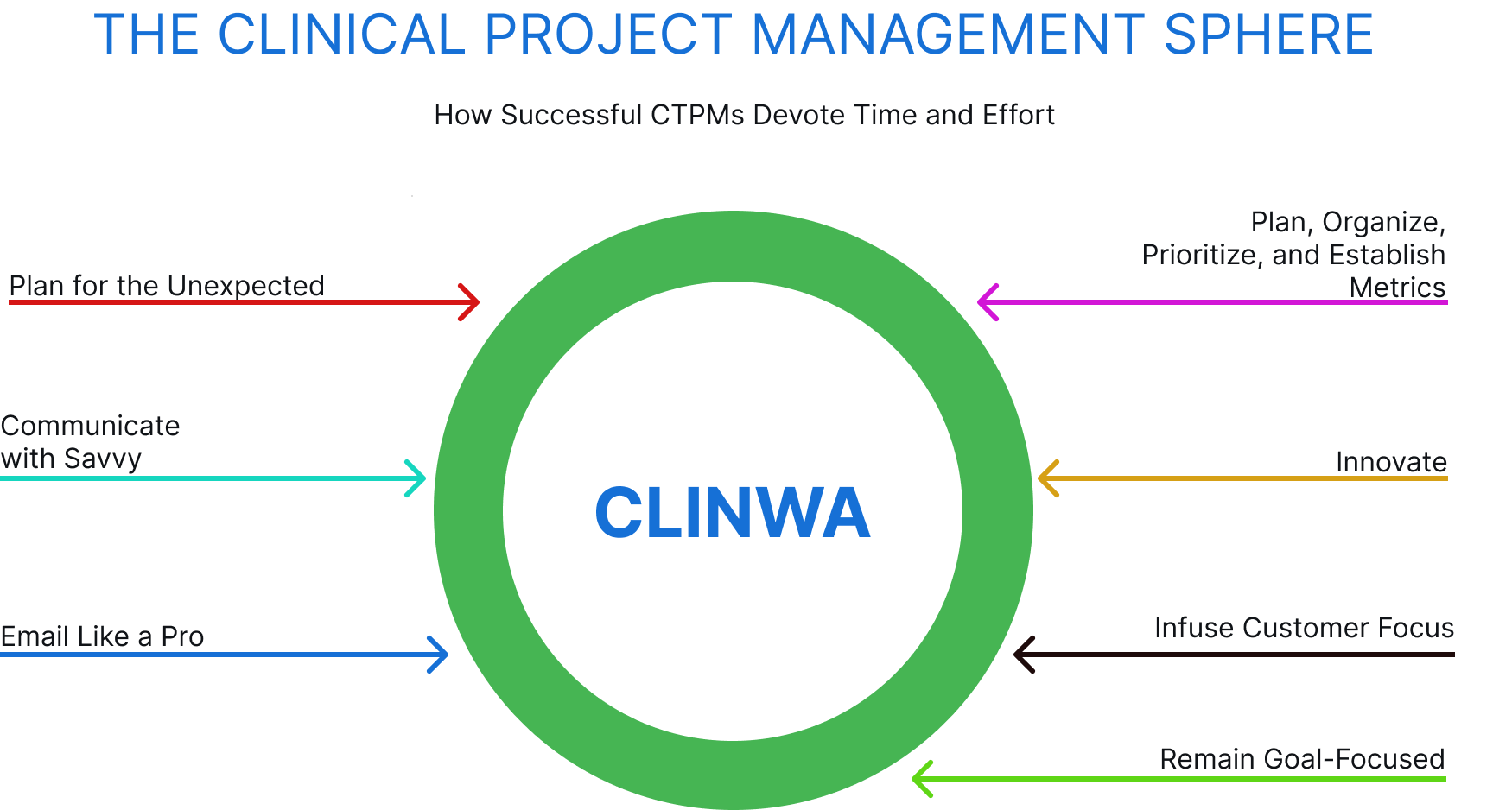
Clinical Project Management Sphere
Here are the seven essential behaviors we adopt to bolster the success of CTPMs while allowing for operation within the company and regulatory edicts.
- Plan, Organize, Prioritize, and Establish Metrics
- Infuse Customer Focus
- Innovate
- Remain Goal-Focused
- Communicate with Savvy
- Email Like a Pro
- Plan for the Unexpected
Contact us to discuss your clinical trial management requirements.
